Ringbio Aerobic Count Plate is based on the ISO/AOAC standard method, which utilizes the dehydrated culture medium to cultivate the aerobic bacteria in 48h to give you a visual and easily-counting result. This count plate is readily usable and no extra culture media is required. The bacteria will grow on the plate with proper incubation. The result can be enumerated with counter.
Key features of Ringbio Aerobic Count Plate
- Visual countable result in 48 hours
- Comparable performance & results with other commercial count plate
- Cost effective and readily availble
- All red colonies shall be counted.
- Applicable for food and beverage industry
- AOAC-RI certified and validated (PTM 062301)
Specifications of this Ringbio Aerobic Count Plate
| Product Code | KGR001 |
|---|---|
| Package Size | 12 tests / pack, customized size available |
| Brand Name | KangarooSci |
| Principle | Culture medium and/or selective culture medium based |
| Enumeration Range | 30-300cfu. Further dilution may require for TNTC result. |
| Incubation | 36oC±1oC for 48h±2h, for aquatic products, please incubate at 30oC±1oC for 72h±3h, |
| Storage Temperature |
|
| Applied Samples | Liquid and/or solid samples. |
| Sample Preparation | Pre-enrichment not required |
| Certification | AOAC-RI, PTM 062301 |
| Reference Standard |
|
| Typical Result of the kit | 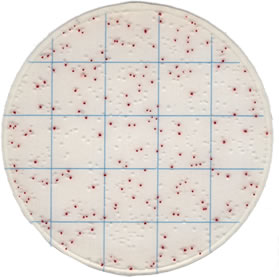 |
Components of Aerobic Count Plate Kit
- 12 count plates in one foil package, further packed in paper box.
Instrument or reagent required to run this aerobic count plate
- Micropipette with 1mL
- pH meter
- NaOH, 1M, for sample pH adjusting
- HCl, 1M, for sample pH adjusting
- Incubator
- Counter
Notice:
- In order to get a precise result, all instrument, devices, and the plastic consumables used in the analysis shall be sterile. Otherwise it will lead to unexpected result.
- Subject to the limitations of the method used in the kit, it is applicable to products intended for human consumption or the feeding of animals. Further internal validation may require if new samples are tested.
Training video on how to use this aerobic count plate
Validation Study Report and Publications
Danping Wei and others, Validation of the KangarooSci® Aerobic Count Plate for the Enumeration of Meosphilic Aerobic Bacteria in Selected Foods and on Stainless Steel Environmental Surfaces: AOAC Performance Tested MethodSM 062301, Journal of AOAC INTERNATIONAL, 2023;, qsad088, https://doi.org/10.1093/jaoacint/qsad088