
COVID-19 Total Antibody Rapid Test Kit
Component of COVID-19 Total Antibody Rapid Test Kit
- COVID-19 Total Antibody Rapid Test Card, 50pcs,
- Sample buffer, 5ml, 2pcs
- Disposable micropipette tips, 50pcs,
- Kit instruction, 1 pc
Significance of tesing IgG/IgM in blood/serum/plasma
- IgM is an antiody produced in the beginning of infection, which indicates an early stage of the disease
- IgG is produced after a period of infection, which indicates the infection is ongoing or was infected.
- Combination of IgG/IgM indicates the infection is ongoing
- Clinical diagnosis shall be determined by RT-PCR/CT scanning.
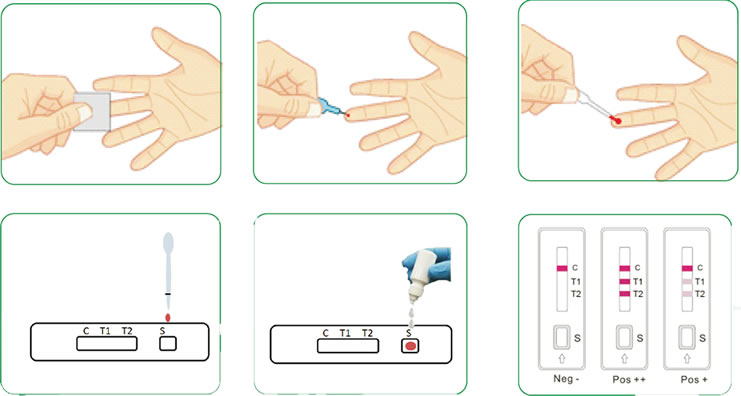
How to use COVID-19 Total Antibody Rapid Test Kit
- Take 10-20ul blood sample (serum, full blood or plasma), add into the sample well on the test card
- Then add 2-3 drops of sample buffer into the well.
- Wait for 10-15min, determine the result according to the kit instruction.
The picture below describes the use of this kit
Precautions on the handling of sample and lab waste
- This kit does not contain any contagious materials, it is safe to ship/use under normal situation.
- All samples are contagious, please handle carefully according to local regulations, or following the guidelines of your local CDC or WHO.
- Used test cards shall be handled carefully as biolab waste, which shall be treated carefully according to guidelines of CDC/WHO.
Limitations of this COVID-19 Total Antibody Rapid Test Kit
This kit is used as aiding tool for the testing of COVID-19, which shall be used in combination with RT-PCR/CT scanning. The results shall be determined by professionals. It is not a home use test. Before licensed by CDC, this kit can only used as research tool, which can not be used in clinical diagnosis of COVID-19.
Coronaviruses (CoV) are a large family of viruses that cause illness, ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV, also known as SARS-CoV-2) is a new strain that has not been previously identified in humans. The disease caused by this novel coronavirus is then named Coronavirus Disease 2019(COVID-19) by WHO.
Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
More information can be found on WHO website, CDC website.
References
Li, Z., Yi, Y., Luo, X., Xiong, N., Liu, Y., Li, S., Sun, R., Wang, Y., Hu, B., Chen, W. and Zhang, Y., Development and Clinical Application of A Rapid IgM‐IgG Combined Antibody Test for SARS‐CoV‐2 Infection Diagnosis. Journal of Medical Virology.



